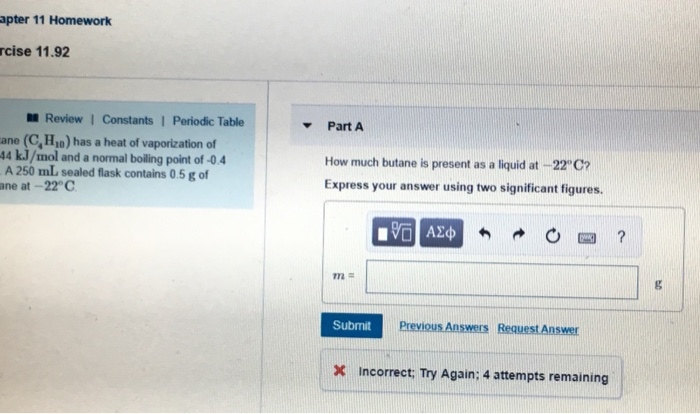
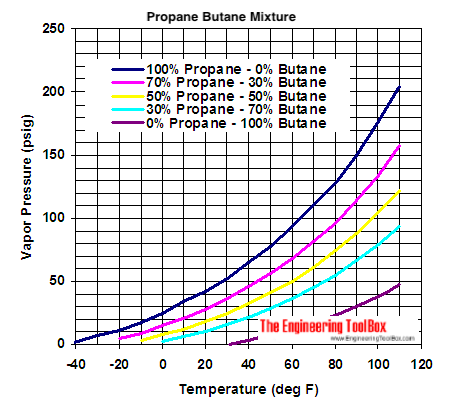
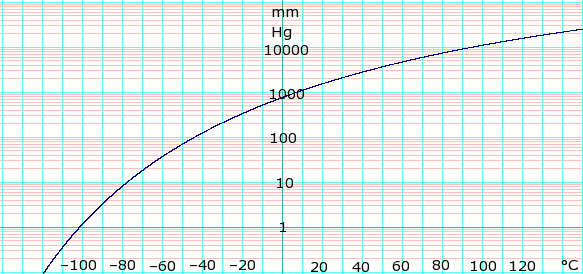
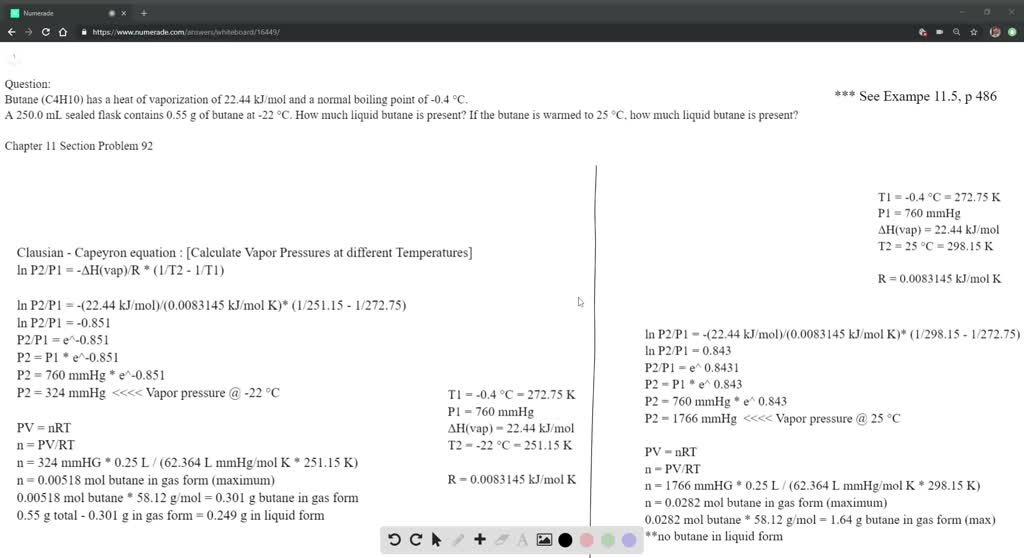
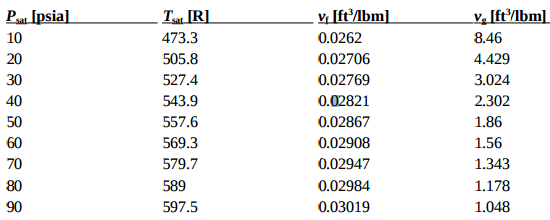
SOLVED: The heat of fusion, Hof, of butane, C4H10, is 4.7 kJ/mol. Calculate the change in entropy, S, when 2.2 g of butane freezes at -138.0°C. The molar mass of butane is
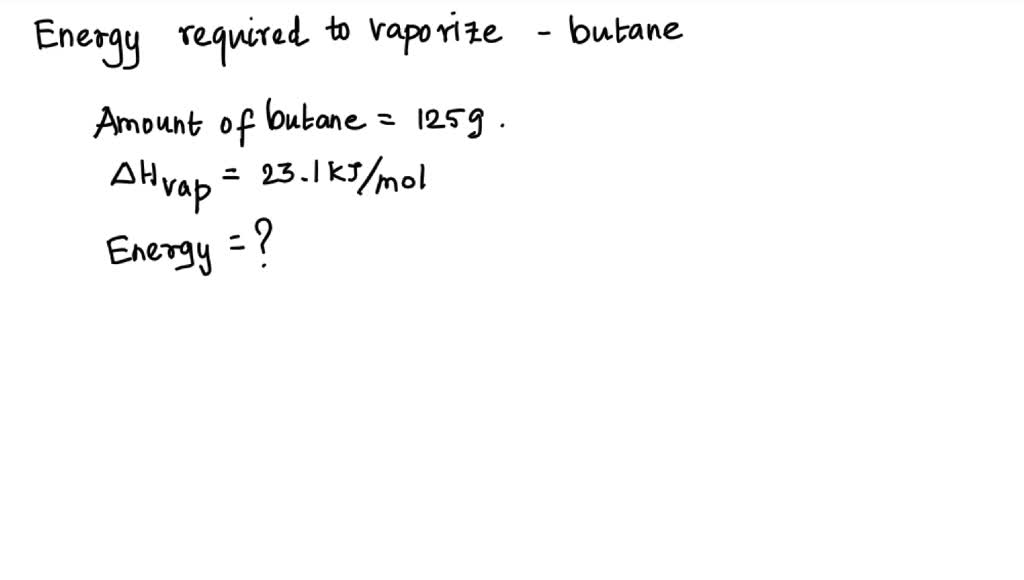
SOLVED: How much energy is required to vaporize 125g of butane at its boiling point? The heat of vaporization for butane is 23.1 kJ/mol.

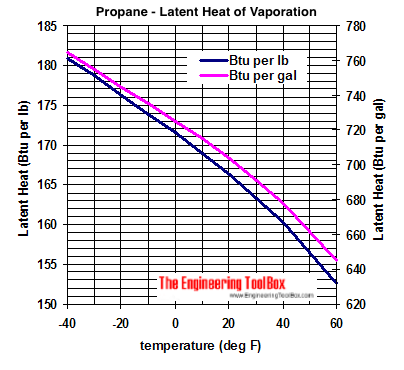
Approximated latent heat of vaporization for (a) butane; (b) isobutane;... | Download Scientific Diagram

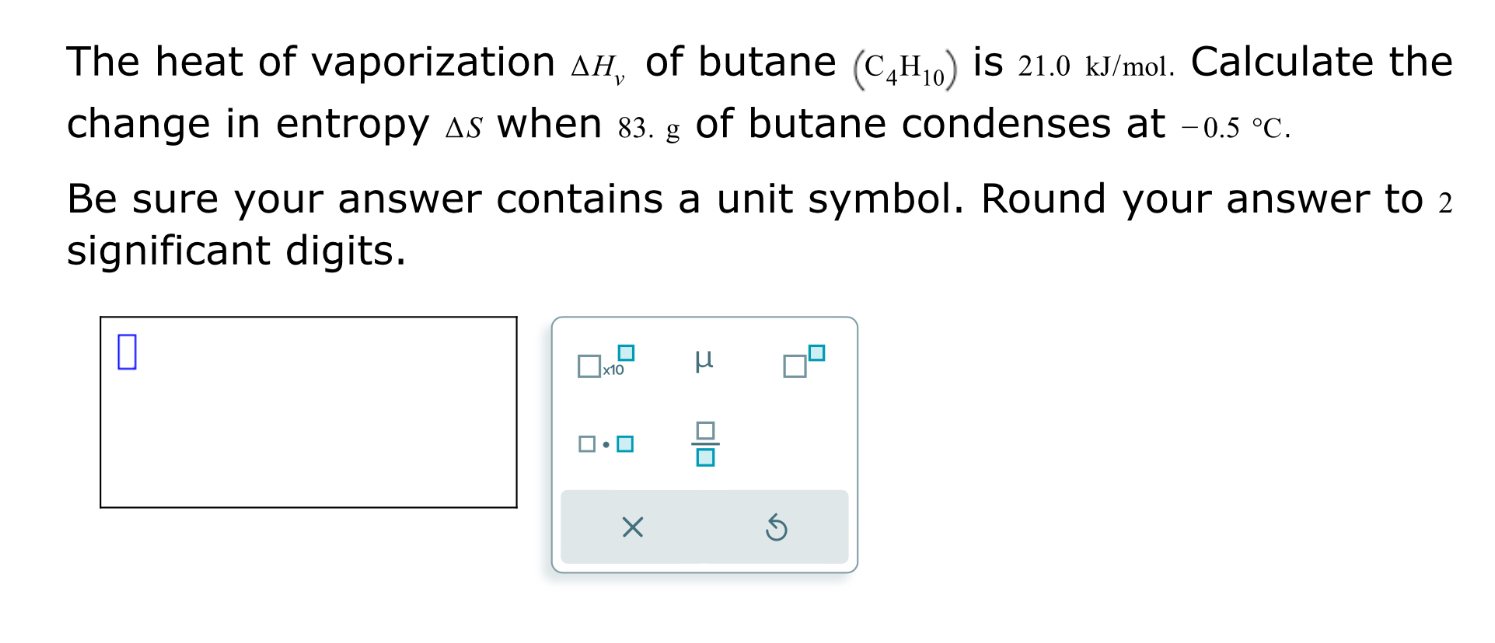
SOLVED: The heat of vaporization ΔHv of butane (C4H10) is 21.0 kJ/mol. Calculate the change in entropy ΔS when 60 g of butane condenses at -0.5°C. Be sure your answer contains a

How much energy is required to vaporize 155 g of butane at its boiling point? the heat of vaporization for - brainly.com



![Latent heat of vaporization for main components of LNG [10]. | Download Table Latent heat of vaporization for main components of LNG [10]. | Download Table](https://www.researchgate.net/publication/330572654/figure/tbl3/AS:718422421803010@1548296661881/Latent-heat-of-vaporization-for-main-components-of-LNG-10.png)